Let’s dive into the X post by Chris Masterjohn (@ChrisMasterjohn) from April 7, 2025, titled “The Worst Longevity Idea Ever Conceived,” where he critiques the use of rapamycin for longevity. The post features an image of a person holding a pill near their mouth, setting the stage for a discussion about pharmaceutical interventions in aging. Masterjohn’s thread is a detailed takedown of rapamycin, a drug popular in the longevity community, arguing that its risks far outweigh its benefits when used perpetually to extend lifespan. Let’s break this down, weave in some context from the provided web results, and add a bit of broader research to flesh out the picture.
The Core Argument: Rapamycin and mTOR Inhibition
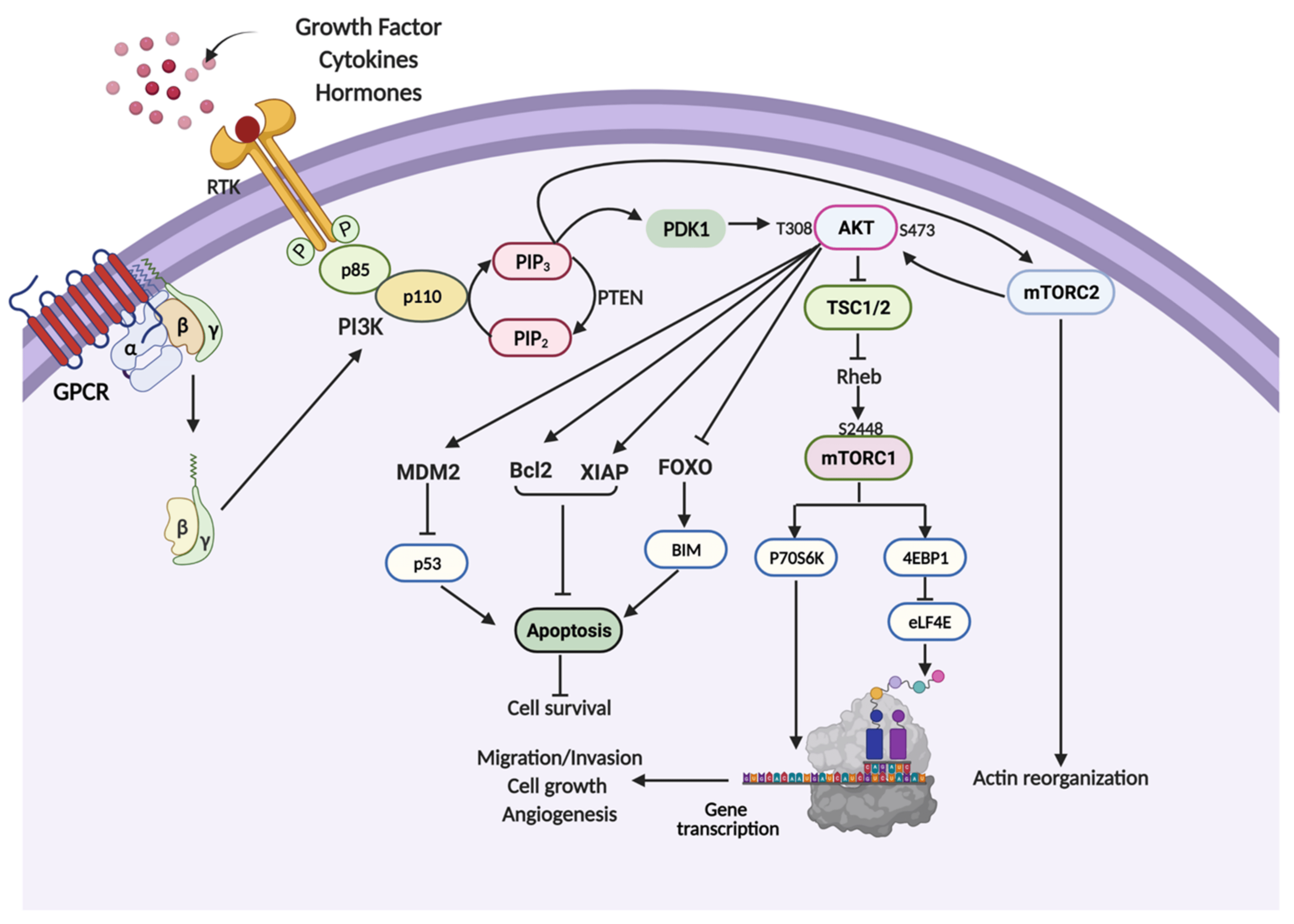
Masterjohn starts with a bold claim: perpetually inhibiting mTOR (mechanistic target of rapamycin) is a terrible idea for longevity. Rapamycin, a drug initially discovered in the 1970s on Easter Island as an antifungal, works by inhibiting mTOR, a protein complex that acts as a nutrient sensor in cells. mTOR regulates growth, protein synthesis, and autophagy (cellular cleanup), processes critical for health and aging. In the longevity space, rapamycin gained fame after a 2009 study in Nature showed it extended lifespan in mice by up to 14%, even when started late in life. This sparked a wave of interest, with off-label use surging among anti-aging enthusiasts. A 2024 systematic review in The Lancet Healthy Longevity noted that 95% of surveyed off-label rapamycin users took it for anti-aging, often at doses like 6 mg weekly.
Masterjohn, however, is skeptical. He argues that while rapamycin can lengthen lifespan in mice, it does so at a “profound expense to healthspan.” He lists severe side effects observed in mouse studies: cataracts, testicular atrophy, impaired glucose metabolism, fatty liver, and heart scarring. These issues appear even at doses below those needed for lifespan extension, and they worsen at higher doses. He also cites rapamycin’s impact on wound healing, noting that it inhibits lipid and collagen synthesis, which can lead to problems like canker sores—a sign, he suggests, of broader tissue damage, potentially even in the brain due to impaired myelin synthesis (a lipid-rich coating essential for nerve function).
mTOR and the Fasting-Feeding Cycle
A key point in Masterjohn’s thread is the biological role of mTOR. He explains that mTOR signals nutrient abundance, ramping up growth and repair when food is plentiful. During fasting, mTOR activity drops, triggering autophagy to “clean house” by breaking down damaged cellular components. Masterjohn likens this to clearing out old clothes from your closet to make room for new ones—fasting cleans out the old, while feeding rebuilds. Rapamycin artificially suppresses mTOR, mimicking a fasting state, but Masterjohn argues this is problematic if done perpetually, especially while eating an abundant diet. The body expects a natural cycling between scarcity and abundance, not a constant “fasting signal” via a drug. He warns that taking rapamycin while eating normally can confuse the body, leading to metabolic chaos—like trying to fool the body, only to have it “fool you” in return.
This perspective aligns with research on mTOR’s dual complexes, mTORC1 and mTORC2, which regulate different aspects of metabolism and growth. A 2019 study in PMC (web ID: 1) notes that mTORC1 inhibition via rapamycin can extend lifespan by reducing protein synthesis and boosting autophagy, but chronic inhibition disrupts the balance. For instance, mTORC2 suppression, which occurs with some mTOR inhibitors, is linked to insulin resistance and reduced lifespan in mice, highlighting the risks of non-specific inhibition.
The Evidence in Mice: Lifespan vs. Healthspan
Masterjohn dives into the mouse studies that have fueled rapamycin’s popularity. He acknowledges that rapamycin extends lifespan in mice, with studies showing increases of 3%, 13%, and 23% at doses of 4.7, 14, and 42 mg/kg of diet, respectively. However, he points out that even the lowest dose, which isn’t statistically significant (P = 0.19), causes significant side effects like testicular degeneration and cataracts. The web results (web ID: 0) confirm that over two dozen studies have shown lifespan extension in mice, starting with a seminal 2009 study by Harrison et al. But Masterjohn emphasizes the trade-off: mice may live longer, but they suffer from scarred hearts, fatty livers, and metabolic issues like impaired glucose metabolism.
A PMC study (web ID: 3) supports Masterjohn’s concerns about glucose metabolism, showing that rapamycin delays glucose clearance across nine inbred mouse strains, with effects ranging from none in one strain to increased insulin sensitivity in another. This variability suggests a genetic component to rapamycin’s metabolic impact, raising questions about its safety in humans with diverse genetic backgrounds. Masterjohn notes that the glucose issues likely stem from taking a fasting-mimicking drug while the mice are not fasting, disrupting normal metabolic cycles.
Intermittent Dosing: A Partial Solution?
Masterjohn explores intermittent dosing as a potential way to mitigate rapamycin’s side effects. In mice, weekly dosing (alternating full dose and no dose) reduces the impairment in glucose metabolism but doesn’t eliminate other issues like heart scarring or testicular atrophy. Interestingly, intermittent dosing extends lifespan in male mice as much as chronic dosing but reduces the benefit in females. He calculates that the 42 mg/kg diet dose, when given every other week, averages to 21 mg/kg, suggesting males might not need more than this for lifespan benefits. For a 70-kg human, this translates to 2, 6.5, or 19 mg/day, but most anti-aging users take 5-7 mg weekly (about 857 µg/day), which is only 43% of the lowest mouse dose—a dose that failed to extend lifespan in mice.
The web results (web ID: 2) note that intermittent dosing (e.g., 2-6 mg weekly) is common in anti-aging protocols and hasn’t shown severe side effects at these low doses. A 2023 PubMed study on tuberous sclerosis complex in mice also found that intermittent rapamycin with drug holidays of over three weeks still prevented epilepsy, suggesting that cycling the drug might balance benefits and risks. However, Masterjohn remains cautious, arguing that even intermittent dosing doesn’t fully address the broader healthspan issues.
Rapamycin’s Broader Implications
Masterjohn touches on a chilling side effect: canker sores, which some in the longevity community see as a sign rapamycin is “working.” He counters that if the drug is “drilling a hole in your mouth,” it might be doing similar damage elsewhere, like in the brain, by impairing myelin synthesis. This concern ties into rapamycin’s known effects on lipid metabolism, which could have long-term neurological implications, though human data on this is limited.
On the flip side, the web results (web ID: 0) highlight some benefits of rapamycin. It prevents lymphoma and certain cancers in transplant patients and is used in cancer therapy (via analogs like everolimus). It also extends lifespan in cancer-prone mice, and some studies suggest it can improve immune status at low doses, contrary to its immunosuppressive effects at high doses. Combining rapamycin with other drugs like metformin (to counter hyperglycemia) or PDE5 inhibitors (for additional anti-aging benefits) is also being explored, as noted in the web results.
Human Translation and Ongoing Research
Masterjohn’s thread raises a critical question: how do mouse studies translate to humans? The doses used in mice are much higher than those typically used by humans for anti-aging (5-7 mg/week vs. the mouse-equivalent of 19 mg/day). A 2024 pilot study in The Lancet Healthy Longevity found that short-term rapamycin treatment (8 weeks) in healthy older adults was safe, but long-term data is scarce. As of July 2023, nine clinical trials listed on ClinicalTrials.gov are investigating rapamycin’s effects on aging-related outcomes, reflecting growing interest despite the risks Masterjohn highlights.
My Take: Balancing Promise and Peril
Masterjohn’s critique is a sobering counterpoint to the hype around rapamycin. He’s right to highlight the healthspan trade-offs—living longer but with cataracts, metabolic issues, and potential brain damage isn’t an appealing deal. His focus on mTOR’s natural cycling resonates with evolutionary biology: our bodies are wired for feast-famine cycles, not constant suppression of growth signals. The mouse data on side effects, especially glucose metabolism, is concerning, and genetic variability in humans could amplify these risks.
That said, the longevity community isn’t entirely wrong to be intrigued. Rapamycin’s ability to extend lifespan in mice is robust, and its anti-cancer effects are well-documented. Intermittent dosing and lower doses might mitigate some risks, as seen in human protocols and emerging research. The ongoing clinical trials will be crucial for clarifying rapamycin’s risk-benefit profile in humans, especially for long-term use.
If you’re considering rapamycin for anti-aging, Masterjohn’s thread is a reminder to proceed with caution. It might have a role in specific contexts—like temporary cycles to clear damaged mitochondria, as he suggests—but perpetual use seems risky based on current evidence. For now, natural fasting-feeding cycles, exercise, and other lifestyle interventions might offer a safer way to harness mTOR’s benefits without the pill.
What do you think? Are you intrigued by rapamycin’s potential, or do Masterjohn’s warnings give you pause?
Leave a Reply